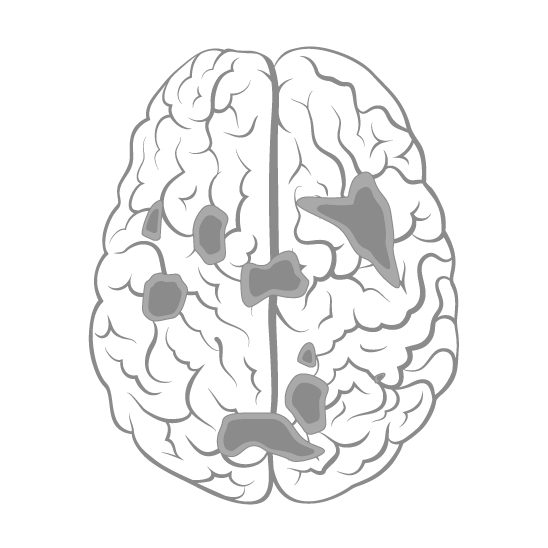
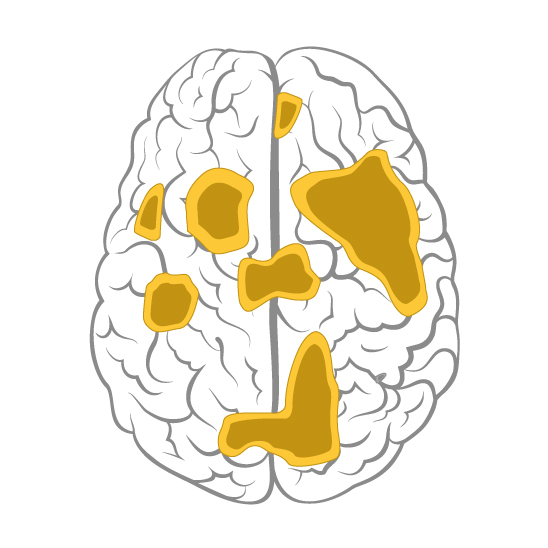
For various decades, electroconvulsive therapy (aka ECT or “shock therapy”) has been considered the last resort for treatment-resistant depression. However, not all patients can tolerate this therapy, not to mention its negative effects on memory, are also a concern.
The advent of transcranial magnetic stimulation (TMS) has been a ray of hope for MDD that failed to improve with medications and psychotherapy. TMS can be used as a stand-alone treatment or as an add-on therapy for treating MDD.
The benefits of TMS for MDD are well-documented. That is why the U.S. Food and Drug Administration (FDA) approved TMS for treatment-resistant depression in 2008.
Since the FDA clearance of these devices in the United States, practitioners have been incorporating TMS into their clinical practice. Both federal and commercial health care insurers now cover rTMS therapy for patients with MDD in several states.
Mounting evidence shows that people with depression who have tried and failed to respond to medications experience a clinically meaningful response with TMS. Several studies have been showing the success and effectiveness of TMS in people with MDD.